Amerimmune Therapeutics: Caspase Inhibitor Project
Rethinking the treatment of novel infectious diseases - cell death as the central problem.
What if there was a simple treatment that would prevent global complications from novel infectious diseases?
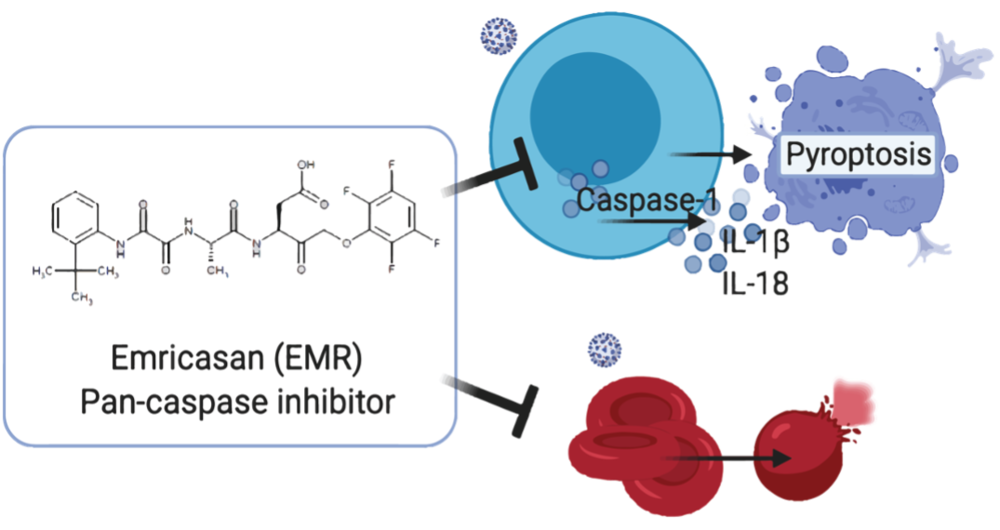
We are researching to see if Emricasan, a molecule that blocks cell death, can be the answer to this question.
Originally formulated by Pfizer, Emricasan is showing promising results in research studies to be effective at protecting blood cells under attack.
Ammerimune now holds commercial licensing rights to this Emricasan formula and we're working hard to bring it to market as an approved COVID-19 treatment.
Before a treatment can be brought to market, it must complete a testing and verification process.
As part of the process, we have already completed Phase I trials and are raising capital for the next phase of testing.
In addition, we have developed at test that measures the chemical in blood cells that emricasan blocks and will also be developing this to identify individuals who may be at higher risk of developing complications of COVID-19, also known as long COVID or post-COVID syndrome.
Caspases in moderate-severe, long COVID-19 Disease
The research paper entitled “Caspases in Moderate-Severe, Long COVID-19 Disease and the Therapeutic Potential of Caspase Inhibitors” has been published in Allergy, the Journal of the European Academy of Allergy and Clinical Immunology, and is currently available electronically at https://onlinelibrary.wiley.com/doi/10.1111/all.14907
The story of Amerimmune begins in early 2020 when a major Washington, D.C.-based hospital system approached nationally renowned immunologist Dr. Oral Alpan with a question…
Why were immunocompromised patients dying from COVID-19?
After months of research Dr. Alpan found the answer hidden deep within our own immune systems. What Dr. Alpan and his Amerimmune team discovered was those immune-compromised patients that died due to a COVID-19 infection all had the “death signal” enzyme. The discovery, first published in the July 2020 issue of the Journal of Hepatology, marked a turning point in how understanding COVID-19 (and numerous other novel diseases) interact with our immune systems.
The interaction of SARS-CoV-2, the novel coronavirus causing the global COVID-19 pandemic, and the human immune system is the focus of intensive research. Although much effort has gone into vaccines, antiviral therapies and dampening the inflammatory response (also referred to as the cytokine storm), the focus is now shifting towards a much different aspect of this disease; cell death induced by SARS CoV2, which can potentially explain the early and late complications seen in COVID19.
Amerimmune, in collaboration with Dr. Raavi Gupta from SUNY Downstate Medical Center and Dr. Lishomva Ndhlovu from Weill Cornell Medicine, demonstrated the impact of caspases in multiple blood cells beyond the acute stage of the disease. There are 12 different caspase molecules in humans which are enzymes that play role in programmed cell death. Of these molecules, caspase-1 can cause bad cell death, also called pyroptosis, that can worsen inflammation (1, 2). The research published in the May issue of “Allergy’, the official Journal of the European Academy of Allergy Asthma and Clinical Immunology “Allergy”, concluded that caspases are elevated in white blood cells of hospitalized COVID19 patients with co-morbidities and persisted into later stages of the disease, also referred to as long COVID (Figure 1) (3). Moreover, the results were not just limited to the elevation of caspase-1 in white blood cells. There was also an elevation of caspase-3 in red blood cells (erythrocytes). These findings have potential implications in understanding the pathogenesis of complications of SARS-CoV2 infection such as extensive blood clot formation, resulting in significant morbidity and mortality (Figure 2). The collaborative work of SUNY Downstate and Weill Cornell Medical Center with Amerimmune demonstrated how in certain individuals regarded as “high risk”, such as asthma, immune deficiencies and chronic sinopulmonary disease, there is already increased baseline caspase-1 expression, potentially setting the stage for complications if they were to be infected with SARS CoV2. All the assays used in this study were developed and validated by Amerimmune. This study now leads to more research opportunities to explore why some individuals develop worse outcomes, whereas some others remain asymptomatic or just have mild disease. Most importantly, the team showed that Emricasan, a pan-caspase inhibitor, can effectively reduce the caspase expression in invitro, paving the way to the use of caspase inhibition as a treatment modality in COVID19.
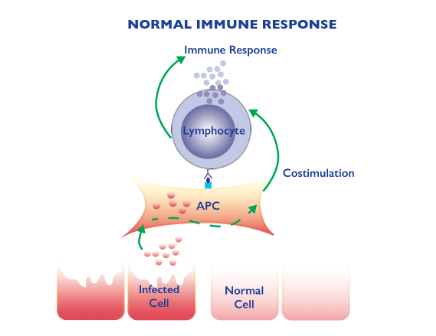
The fact that the same SARS CoV2 virus causing asymptomatic infection in some people but more complicated outcomes in others clearly puts the “host” at the center of the problem. Immunity to viruses are triggered by infected tissues giving alarm signals that activates antigen presenting cells (4). These cells engulf the virus and then induce immunity by activating white blood cells called lymphocytes. There are two types of white blood cells; B cell s that make antibodies and T cells that play role in cellular immunity. (Figure 3). In general this immune response results in clearing of the virus (e.g. by antibodies) and virus infected cells (by T cells). Healthy cells and tissues are spared by the immune response.
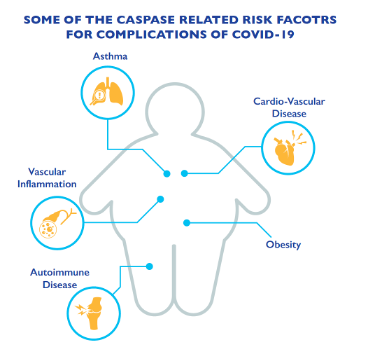
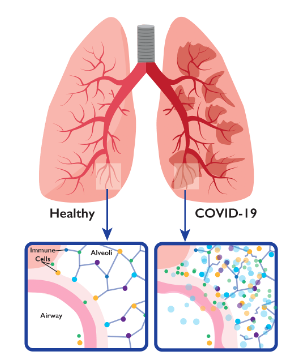
In the setting of COVID19, the finding of increase in caspase-1 molecule in lymphocytes is an evidence for cell death. This can result in the demise of these key elements of the immune system that protects against the viral infection. COVID19 patients who present to the hospital with low lymphocyte counts have worse outcomes then those with normal counts, which provides further support for our findings. This bad cell death can subsequently lead to the damage of un-infected cells resulting in complications that are seen in COVID19. (Figure 4). Elevated baseline levels of caspases in in high risk conditions such as asthma, immune deficiencies, cardiovascular disease, obesity, etc.. may be the reason why such individuals are impacted harder with COVID19.
One can also envision, at the conclusion of COVID19, with all the cell death happening in the body, the individual can be left with an imbalance of the immune system cells in tissues (e.g. lung, heart, grain, etc..). This imbalance now has been shown in multiple studies and can potentially explain long COVID (Figure 5). An approach with a caspase inhibitor at an earlier stage of COVID19, before extensive cell death happens, may be the key to reducing disease severity as well as prevention of complications in patients infected with SARS CoV2.
A Phase 1 clinical trial of Emricasan in mild Covid19 is underway at S.U.N.Y. Downstate Medical Center with the leadership of Dr Raavi Gupta as the principal investigator. Enrollment is planned to be complete in late May 2021. “Emricasan is a small molecule that is administered orally with the potential of access to multiple tissues and organs. This therapeutic modality could address the events at the initiation of the COVID19 process, and may have the potential to prevent the down-stream complications” said Oral Alpan, MD, CEO of Amerimmune. The findings from this important research support the potential of emricasan as a treatment option for COVID-19 in the acute and long-haul phases of the disease,” said Richard Pascoe, President and CEO of Histogen. “We look forward to the completion of our ongoing Phase 1 Study in mild-moderate symptomatic COVID-19 patients at SUNY Downstate Medical Center. Importantly, we have seen no adverse events to date in the study and we anticipate completion of the study in Q2 2021.”
Management Team
Dr. Oral Alpan, MD
Chief Executive Officer & Chief Medical Office, Amerimmune LLC
Oral Alpan, MD, is the Lab and Medical director of Amerimmune, LLC, CEO and Medical Director at O&O Alpan, LLC in Fairfax, Virginia, and Associate Professor of Pediatrics at Virginia Commonwealth University’s Inova Campus in Falls Church. He received his medical degree from Hacettepe University Faculty of Medicine in Ankara, Turkey. Dr. Alpan continued his education with an internship and residency in Pediatrics at Stony Brook University in New York and a fellowship in Allergy/Immunology at the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland. Board-certified in Allergy and Immunology, Dr. Alpan is a member of the American Academy of Allergy, Asthma & Immunology. He is a Principal Investigator on numerous research studies in the field of immunology asthma and allergic disorders, and he has published a number of articles and abstracts in peer-reviewed journals.
Dr. Alpan can be reached at oalpan@amerimmune.com.
Dan Chambers
Vice President of Business Development, Amerimmune LLC
Dan is a co-founder of Amerimmune LLC and he serves as Amerimmune’s Vice President of Business Development. Dan is a patent and licensing attorney with more than 25 years of experience in protecting and monetizing various life science technologies, including pharmaceuticals (proteins and small molecules), medical devices, and molecular diagnostics. He has served in various capacities in-house (e.g., Amgen, Viagene) as well as in private practice (e.g., Wilson Sonsini, Borbeck, Lyon & Lyon).
Dan can be reached at: dchambers@amerimmune.com.
Tom Byrne
Chief Legal Counsel, Amerimmune LLC
Thomas Byrne is an experienced pharmaceutical R&D executive with special expertise in related intellectual property law. His experience includes in-house counsel positions within both Genentech and Amgen. While at Amgen, he co-invented the erythropoiesis-stimulating agent Aranesp®. From 1992-2000 he was a partner in the intellectual property law firm of Nixon and Vanderhye P.C. Since 2000, Mr. Byrne has provided expert legal counsel for numerous start-up biotechnology and pharmaceutical companies on intellectual property, contract and business issues.
Mr. Byrne holds BS degrees in Chemical Engineering and Nuclear Engineering from the University of Virginia, an MS degree in Biochemical Engineering from Yale University, and a JD degree from the University of Virginia.
Ivan Santos
Chief Financial Officer, Amerimmune LLC
Ivan has over 30 years of experience in finance and operations. He did his undergraduate work at the University of Maryland and graduate work at Georgetown University and George Mason University. He worked in larger organizations at the beginning of his career, including Northrop Grumman and Accenture. Later, he transitioned to be the Chief Financial Officer for other organizations taking most of them through hyper-growth and custom exit strategies. Ivan has been with Amerimmune since 2016 developing it’s corporate structure and market offerings.
Ivan can be reached at isantos@amerimmune.com